In the early years of my training to be a pediatric infectious disease physician, I was able to see first hand how vaccines really save lives and impact the welfare of children. I completed my medical training in Turkey and was part of research groups, back in 2008 and 2009, looking into central nervous system infections. We collected samples of cerebral spinal fluid from different hospitals in Turkey and the findings from these studies contributed to two vaccines being introduced into the country’s routine child immunisation. That was incredible.
Then, I travelled to the U.S. to complete my doctorate in epidemiology of vaccines and vaccine preventable diseases. I am a strong believer that vaccines are perhaps only second to clean drinking water when it comes to creating a thriving, healthy society. I also advocate for vaccines being available and accessible, independent of geographic location or socioeconomic status. So, my involvement with COVID-19 vaccines since the pandemic began really came as an extension of the type of work I was already involved in.
I was actually one of the investigators for the phase 1 Moderna mRNA-1273 trials among adults back in March 2020 that was conducted in collaboration with National Institute of Health (NIH). This trial started five days after the World Health Organisation (WHO) announced the pandemic and a participant in our trial consented to become one of the first human beings to receive the Moderna mRNA-1273 vaccine. Nine months after my conversation with that young man, I got the same vaccine in my own arm.
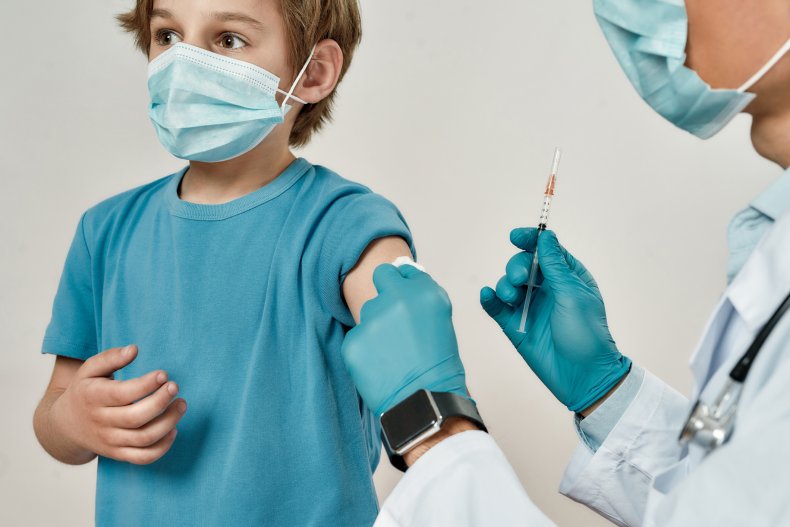
In mid-March 2021, Moderna in collaboration with the NIH started its mRNA vaccine trials across the 6 months to 12 year old age group and at Yale School of Medicine, we started enrolling in this same age group on May 15. It’s exactly the same vaccine that millions of adults have now received, and many teenagers have already received in other clinical trials, but the dose may differ based on the results of the study.
I have been happily surprised with the interest parents have had in being part of our study. New Haven, where Yale is located, has one of the most diverse populations in the U.S. We need to ensure that those in the study are a reflection of that diversity. Unfortunately with the experiences minorities have had in research in the U.S.—and many other places in the world—there are concerns. And I understand that. So, ongoing work, along with the Yale Cultural Ambassadors program, is happening to build that trust within minority communities. But we have had hundreds of emails and calls from parents who are willing to learn about, and be part of, our study. In fact, we have a waitlist for this trial.
I believe there are a couple of reasons for this. Of course, everyone wants the best for their child, but the perception of what a vaccine can give you changes as the infection experience changes.
Many people have now lost grandparents, friends and neighbours to COVID-19. And, as the pandemic evolved we have learned that children do get infected and transmit COVID to other children and adults. Even more worryingly, children with COVID-19 can develop a very serious condition called Multisystem Inflammatory Syndrome (MISC).
I have seen patients as young as six months of age in our intensive care unit (ICU) with MISC. Most of them find that they recover with supportive therapy, but we have had patients who have had breathing tubes because they could not sustain respiratory function.
Getty/iStock
The other impact of COVID-19 in children is long-COVID. I have seen children who had mild COVID-19 telling me a few months later that they can’t run as fast as they did before contracting the virus. Or that they cannot catch their breath while playing sports, or cannot concentrate in class. The rate of infection and mortality among younger children is fortunately lower compared to older adults, but it is significant. Any disease that brings our children to the hospitals and ICUs is significant.
So, it’s clear that these trials are important and necessary. This will help us to have the information we need when we have to decide if and how these vaccines will be implemented in this age group. The epidemiology of COVID-19 has been evolving, and we don’t know how it will evolve in the future. So, we need to be prepared. The study I’m working on follows the “age de-escalation, dose escalation” design. We start with the older age group (within the 6 months to 12 year old age bracket) and provide them with a lower dose of the vaccine, which is administered through a tiny needle that goes into the arm muscle. Then, we observe them for a period of weeks to check the safety of the medicine. Once we have compiled that data and there are no safety concerns, we increase the dose in that same age group. This is one of the unique features in pediatric vaccine trials compared to adults. We have many safety checkpoints. If there are no safety concerns the trials will then move down to a lower age group and begin with a lower dose of the vaccine.
I am aware that parents may have concerns around the amount of blood draws happening in pediatric vaccine trials. With the adult study we had multiple blood draws to check the safety and how it was protecting the participants. Adults can tolerate blood draws more easily, but this procedure can be traumatic for the kids or parents to go through. We do need that data and to observe the blood cells to make sure the vaccine is safe and effective but the amount of blood drawn is based on weight and we only schedule a maximum of three blood draws per child over the 12 month period the children are in study.
Children and families who opted to be in this study have been excited to be part of an effort to find a safe and effective vaccine against COVID-19 in this age group. All the families involved decided to enroll their children after they have done extensive research on the study and having detailed conversations with their younger ones. Children who have been involved in our study so far have told us that they are excited for the pandemic to end and about being part of finding a vaccine that can help other kids. This is really important to me as well. My primary duty as an infectious disease physician is working with childhood transplant patients, so I appreciate how herd immunity among their peers will help them thrive as their immune systems are compromised.

Anthony DeCarlo/Yale Medicine
So far, in Moderna trials across the U.S. there has been nothing that dictated stopping the study or changing the study design, product or age group. If everything goes as planned we will have data for the U.S. Food and Drug Administration (FDA) to review later in 2021 or early 2022.
This is a vaccine that we are testing for the first time in this age group, so there is always an unknown, but there is very strong, clear-cut data from the past 10-12 months showing the efficacy of these vaccines.
This work is what I have wanted to do and what I have tried to be prepared for since I was younger. Although I would not want to have the experience of a pandemic again for many reasons, I think it has shown that science and research benefits each and every one of us in daily life. It’s clear it’s not a theory that science will help you at some point. My hope now is that we continue scientific research and work hard to ensure these vaccines are available for anyone.
The past year has also given me a deeper appreciation for the collaboration of the scientific community; for the physicians and our patients. But most importantly, for our participants. They are volunteering for these trials and letting us use their information and samples to test what we’re looking for. What they are doing may not seem to benefit them immediately, but in the longer term they are doing so much for their communities.
Inci Yildirim, MD, PhD, a vaccinologist and pediatric infectious disease specialist at Yale Medicine and an associate professor of pediatrics at the Yale School of Medicine.
All views expressed in this article are the author’s own.
As told to Jenny Haward.
Newsweek, in partnership with NewsGuard, is dedicated to providing accurate and verifiable vaccine and health information. With NewsGuard’s HealthGuard browser extension, users can verify if a website is a trustworthy source of health information. Visit the Newsweek VaxFacts website to learn more and to download the HealthGuard browser extension.
No comments:
Post a Comment